how many neutrons does bromine have|How to find the Number of Protons, Electrons, Neutrons for : Cebu The number of neutrons in an atom can be determined by the difference between the atomic mass and the number of protons. The difference between the mass number of the bromine atom and the number of protons is . Protest slogans have marked green campaigns since "Give Earth a Chance" banners flew at the first Earth Day march in 1970. As climate crisis protests have intensified, some powerful phrases are .
PH0 · Protons Neutrons & Electrons of All Elements (List
PH1 · How to find the Number of Protons, Electrons, Neutrons for
PH2 · How Many Protons, Neutrons and Electrons Does
PH3 · Chemical Elements.com
PH4 · Bromine (Br)
PH5 · Bromine
For your next United flight, use this seating chart to get the most comfortable seats, legroom, and recline on . United Boeing 777-200 (772) Layout 5. Note: There are 5 versions of this aircraft. Seat Map; Info; Photos; Click any seat for more information .
how many neutrons does bromine have*******BlockElements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Atomic numberThe number of protons in an atom. The number of neutrons in an atom can be determined by the difference between the atomic mass and the number of protons. The difference between the mass number of the bromine atom and the number of protons is .
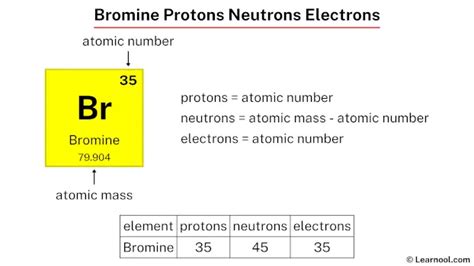
Bromine is a chemical element with atomic number 35 and 35 protons and electrons. It has two natural isotopes, 79 Br and 81 Br, with 44 .
March 23, 2023 Jay. Protons, neutrons and electrons of all elements are mentioned in the table below. Free Gift for you: Interactive Periodic Table. Let me tell you how this Interactive Periodic Table will help you in your studies. 1). . Bromine is a chemical element with atomic number 35 and atomic mass 79.904 u. It has two stable isotopes with 79 and 81 neutrons each.Bromine is the 35th element in the periodic table and has a symbol of Br and atomic number of 35. It has an atomic weight of 79.904 and a mass number of 79. Bromine has thirty-five .
In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Bromine (Br). From the Periodi.Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of .
Symbol: Br. Atomic Number: 35. Atomic Mass: 79.904 amu. Melting Point: -7.2 °C (265.95 K, 19.04 °F) Boiling Point: 58.78 °C (331.93 K, 137.804 °F) Number of Protons/Electrons: 35. .how many neutrons does bromine have How to find the Number of Protons, Electrons, Neutrons for Natural bromine is a mixture of two stable isotopes: bromine-79 (50.54 percent) and bromine-81 (49.46 percent). Of the 17 known radioactive isotope s of the element, bromine-77 has the longest half-life (57 hours).
In 1840, bromine was discovered to have some advantages over the previously used iodine vapor to create the light sensitive silver halide layer in daguerreotypy. [24] . (t 1/2 = 35.28 h), which may be produced from the neutron activation of . Mass number (approx. 79) - Atomic number (35) = Number of neutrons (44) So, this isotope of bromine would have 44 neutrons. However, since there are two natural isotopes of bromine, the number of neutrons in the other isotope would be different, determined in the same way using its specific mass number.How many protons and neutrons does it contain, and what is its charge? [reveal-answer q=”488361″]Show Answer[/reveal-answer] . Bromine has two isotopes 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu) and .
A neutron is one of the subatomic particles that make up matter. In the universe, neutrons are abundant, making up more than half of all visible matter.It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron.The neutron has a mean square radius of about 0.8×10−15 .
All other isotopes have half-lives under 1 hour, many less than one second. Chlorine-35 is composed of 17 protons, 18 neutrons, and 17 electrons. Chlorine-37 is composed of 17 protons, 20 neutrons, and 17 electrons. Chlorine-36 is composed of 17 .
Name: Bromine Symbol: Br Atomic Number: 35 Atomic Mass: 79.904 amu Melting Point:-7.2 °C (265.95 K, 19.04 °F) Boiling Point: 58.78 °C (331.93 K, 137.804 °F) Number of Protons/Electrons: 35 Number of Neutrons: 45 Classification: Halogen Crystal Structure: Orthorhombic Density @ 293 K: 3.119 g/cm 3 Color: Red Atomic Structure : Number of .
Protons, neutrons and electrons of all elements are mentioned in the table below (You will get the List + Shell diagram of all the elements.) . Bromine has 35 protons, 45 neutrons and 35 electrons: 36: Krypton has 36 protons, 48 neutrons and 36 electrons: 37:
The following table shows the atomic nuclei that are isotonic (same neutron number N = 46) and isobaric (same nucleon number A = 81) with Bromine-81. Naturally occurring isotopes are marked in green; light green = naturally occurring radionuclides. Z Isotone N = 46 Isobar A = 81; 25: 71 Mn: 26: 72 Fe: 27: 73 Co: 28: 74 Ni: 29: The bromine vapors have a very pungent odour and it can also irritate our eyes. The melting point of bromine is -7.2 °C and its boiling point is 58.8 °C. The atomic mass of bromine is 79.904 u and its density is 3.12 g/cm 3 .

bromine; Solutions. Hg (transition metal)- has 80 electrons, 80 protons, and 121 neutrons; Pt (transition metal)- has 78 electrons, 78 protons, and 117 neutrons; Br (halogen)- has 35 electrons, 35 protons, and 45 neutrons
How many protons does the isotope 105B have? a. 5 b. 10 c. 6 d. 15; How many neutrons are in the nucleus of the atom 3517Cl? a. 35 b. 17 c. 18 d. 52; What isotope of lead (Pb) has 126 neutrons? How many protons and electrons does it have? How many protons, neutrons, and electrons are in Li+ with an atomic mass of 6?It is defined as being the charge that an atom would have if all bonds were ionic. Uncombined elements have an oxidation state of 0. The sum of the oxidation states within a compound or ion must equal the overall charge. Isotopes. Atoms of the same element with different numbers of neutrons. Key for isotopeshow many neutrons does bromine haveBromine (35 Br) has two stable isotopes, 79 Br and 81 Br, and 35 known radioisotopes, the most stable of which is 77 Br, with a half-life of 57.036 hours.. Like the radioactive isotopes of iodine, radioisotopes of bromine, collectively radiobromine, can be used to label biomolecules for nuclear medicine; for example, the positron emitters 75 Br and 76 Br can be used for positron emission .
Valence electrons found in the s and p orbitals of the highest energy. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5 the valence electrons are in the 4s and 4p orbitals giving Bromine 7 valence electrons. I hope this was helpful. SMARTERTEACHERHow many protons and neutrons are in the nucleus of a bromine atom? There are 35 protons and 35 electrons in the elementary Bromine, BrThe number of neutrons however depends on the isotope mass .
How to find the Number of Protons, Electrons, Neutrons for For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons.
For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons.
PNP Provident Fund offers various loan services for its members, such as salary loan, emergency loan, and educational loan. Learn more about the features and benefits of each loan and how to apply online.
how many neutrons does bromine have|How to find the Number of Protons, Electrons, Neutrons for